Ammonia is produced commercially by the Haber reaction, a groundbreaking process that has revolutionized agriculture, industry, and the very fabric of our society. This reaction, discovered by Fritz Haber and perfected by Carl Bosch, has enabled the mass production of ammonia, a crucial component in fertilizers, explosives, and countless other products.
The Haber reaction is a chemical process that combines nitrogen and hydrogen gases to form ammonia. This reaction requires high temperatures, pressures, and a catalyst to proceed efficiently. The catalyst used in the Haber reaction is typically iron oxide promoted with other metals.
Haber Reaction Overview: Ammonia Is Produced Commercially By The Haber Reaction
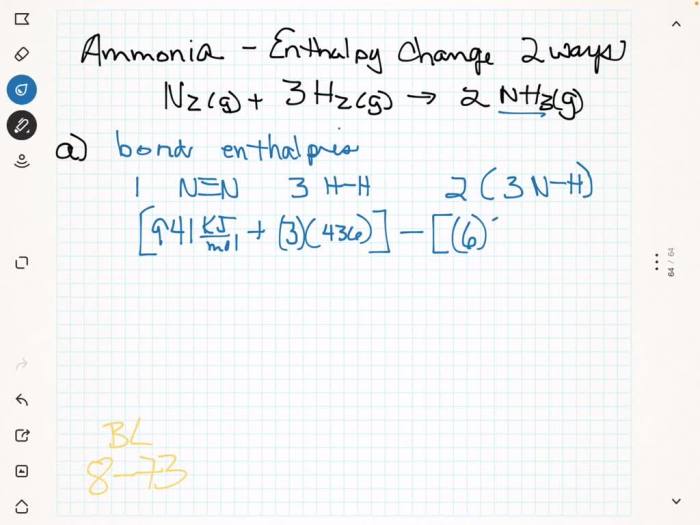
The Haber reaction, also known as the Haber-Bosch process, is a landmark chemical reaction that revolutionized ammonia production and shaped the modern agricultural industry. It is a pivotal process in the synthesis of ammonia (NH3), a crucial component in the production of fertilizers and various chemical products.
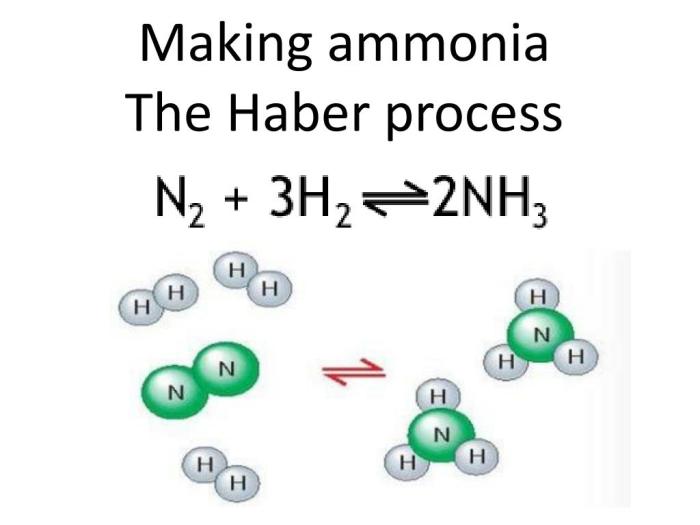
The Haber reaction is an exothermic reaction between nitrogen (N2) and hydrogen (H2) gases, yielding ammonia (NH3):
N2(g) + 3H2(g) → 2NH3(g) + heat
This reaction occurs under specific conditions of high temperature (400-500°C), high pressure (200-300 atmospheres), and in the presence of a catalyst, typically iron oxide with promoters.
Industrial Applications of Ammonia
Ammonia has a wide range of industrial applications, with its primary use being in the production of fertilizers. Ammonia-based fertilizers provide nitrogen, an essential nutrient for plant growth, and play a vital role in increasing crop yields. Ammonia is also a precursor for various chemicals, including nitric acid, urea, and ammonium salts, which are used in the manufacturing of explosives, plastics, and pharmaceuticals.
Beyond fertilizers, ammonia finds applications in refrigeration systems as a refrigerant and in the production of textiles, dyes, and cleaning agents. Its diverse industrial uses have made ammonia a cornerstone of the chemical industry.
Catalysts in the Haber Reaction
Catalysts play a crucial role in the Haber reaction, enabling the conversion of nitrogen and hydrogen gases into ammonia at a commercially viable rate. The catalyst used in the Haber process is typically iron oxide (Fe2O3) promoted with small amounts of other metals or oxides, such as potassium oxide (K2O) or aluminum oxide (Al2O3).
The catalyst provides an active surface for the reaction to occur, facilitating the breaking of the strong triple bond in nitrogen (N2) and the subsequent formation of ammonia (NH3). The catalyst lowers the activation energy of the reaction, making it more likely to proceed at the given temperature and pressure.
Challenges and Future Directions, Ammonia is produced commercially by the haber reaction
The Haber reaction is an energy-intensive process, consuming large amounts of natural gas as a hydrogen source. Additionally, the production of ammonia contributes to greenhouse gas emissions, primarily through the release of carbon dioxide (CO2) during hydrogen production.
Ongoing research and development efforts focus on optimizing the Haber process to reduce energy consumption and environmental impact. Alternative catalysts and reaction conditions are being explored to improve the efficiency and sustainability of ammonia production.
Environmental Impact of the Haber Reaction
The Haber reaction has a significant environmental impact, primarily due to the energy consumption and greenhouse gas emissions associated with hydrogen production. The process also consumes large amounts of water, which can strain water resources in some regions.
Strategies to mitigate the environmental impact of the Haber reaction include developing more energy-efficient processes, utilizing renewable energy sources for hydrogen production, and implementing carbon capture and storage technologies to reduce greenhouse gas emissions.
Historical Significance of the Haber Reaction
The Haber reaction was first developed by German chemist Fritz Haber in 1908. In 1913, Carl Bosch successfully scaled up the process for industrial production, earning them the Nobel Prize in Chemistry in 1918.
The Haber-Bosch process revolutionized agriculture by providing a reliable and cost-effective source of nitrogen fertilizers, enabling the production of large quantities of food to support a growing population. It laid the foundation for the modern chemical industry and continues to be essential for global food security.
Clarifying Questions
What is the Haber reaction?
The Haber reaction is a chemical process that combines nitrogen and hydrogen gases to form ammonia.
Who discovered the Haber reaction?
Fritz Haber discovered the Haber reaction in 1908.
What is the catalyst used in the Haber reaction?
The catalyst used in the Haber reaction is typically iron oxide promoted with other metals.
What are the industrial applications of ammonia?
Ammonia is used in the production of fertilizers, explosives, and a variety of other products.