Find the limiting reactant for each initial amount of reactants – The identification of the limiting reactant, a crucial step in chemical reactions, takes center stage in this exploration. Understanding this concept enables chemists to predict reaction outcomes and optimize chemical processes.
This discourse delves into the methods for determining the limiting reactant, exploring stoichiometry, mole ratios, and their significance in unraveling reaction dynamics.
Introduction
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, thereby limiting the amount of product that can be formed. Identifying the limiting reactant is crucial for predicting the maximum yield of a reaction and understanding its stoichiometry.
Determining the limiting reactant requires an understanding of stoichiometry and mole ratios. Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction, while mole ratios are the ratios of the moles of reactants and products involved in a reaction.
Methods for Determining Limiting Reactant
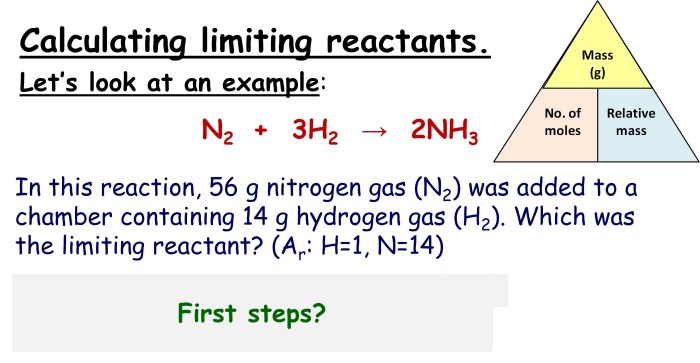
To determine the limiting reactant, follow these steps:
- Balance the chemical equation to determine the mole ratios of the reactants and products.
- Convert the given amounts of reactants to moles using their respective molar masses.
- Divide the number of moles of each reactant by its stoichiometric coefficient in the balanced equation.
- The reactant with the smallest value obtained in step 3 is the limiting reactant.
Examples and Applications: Find The Limiting Reactant For Each Initial Amount Of Reactants
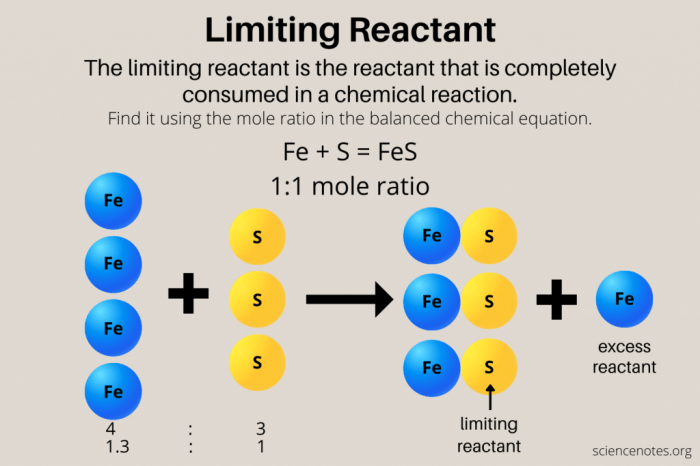
Consider the reaction between hydrogen (H 2) and oxygen (O 2) to form water (H 2O):
| Reactant | Initial Amount (mol) | Mole Ratio | Moles of H2O Formed |
|---|---|---|---|
| H2 | 2 | 2 | 1 |
| O2 | 1 | 1 | 1 |
Since 1 mole of O 2reacts with 2 moles of H 2, and we have only 1 mole of O 2, it is the limiting reactant. This means that all of the O 2will be consumed, and only 1 mole of H 2O will be formed.
Advanced Considerations
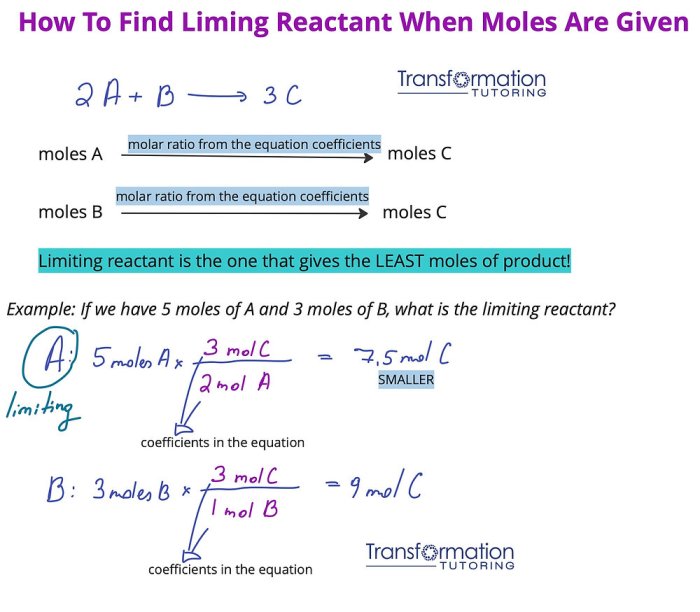
In reactions involving multiple limiting reactants, the overall limiting reactant is the one that produces the least amount of product. Excess reactants do not affect the identity of the limiting reactant but can influence the reaction rate and product yield.
Limiting reactant calculations are essential in chemical synthesis and industrial processes. They help optimize reaction conditions, minimize waste, and ensure efficient production of desired products.
Expert Answers
What is the significance of identifying the limiting reactant?
Determining the limiting reactant allows chemists to predict the maximum amount of product that can be formed in a reaction, optimize reactant ratios, and minimize waste.
How do mole ratios aid in identifying the limiting reactant?
Mole ratios, derived from the balanced chemical equation, provide the stoichiometric relationship between reactants. By comparing the initial moles of reactants to the mole ratios, the reactant present in the smallest relative amount can be identified as the limiting reactant.
What are the implications of excess reactants in a reaction?
Excess reactants do not participate in the reaction beyond the point where the limiting reactant is consumed. They remain unreacted and can be recovered or utilized in subsequent reactions.