Unit chemical quantities molar mass 2 step ws 3 introduces the fundamental concepts of unit chemical quantities and molar mass, providing a comprehensive overview of their applications in chemistry. This guide delves into the calculations, applications, and real-world examples of these important concepts, empowering students with a deep understanding of their significance in the field.
Unit chemical quantities, such as the mole, represent specific amounts of substances, while molar mass, expressed in grams per mole, determines the mass of a given substance. Understanding these concepts is crucial for stoichiometry and chemical reactions, enabling chemists to determine the quantities of reactants and products involved in chemical processes.
Unit Chemical Quantities and Molar Mass
Unit chemical quantities are fundamental measurements used to quantify the amount of a substance in chemistry. The most common unit chemical quantity is the mole, which represents a specific number of elementary entities (atoms, molecules, ions, or electrons). Molar mass is a measure of the mass of one mole of a substance and is expressed in grams per mole (g/mol).
Calculations Involving Unit Chemical Quantities and Molar Mass

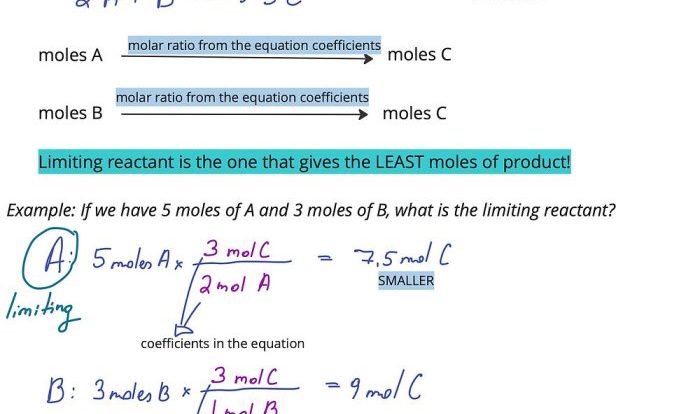
Calculations involving unit chemical quantities and molar mass are essential for stoichiometry and chemical reactions. These calculations allow us to determine the number of moles, mass, or volume of a substance involved in a chemical reaction.
Examples of Calculations
- Converting mass to moles: mass (g) / molar mass (g/mol) = moles
- Converting moles to mass: moles x molar mass (g/mol) = mass (g)
- Calculating the number of atoms or molecules in a sample: moles x Avogadro’s number (6.022 x 10 23mol -1) = number of atoms or molecules
Steps in Calculating Molar Mass
- Determine the chemical formula of the substance.
- Look up the atomic masses of each element in the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the formula.
- Add up the masses of all the elements to obtain the molar mass.
Practice Problems and Solutions, Unit chemical quantities molar mass 2 step ws 3
1. Calculate the molar mass of sodium chloride (NaCl).
Solution:Atomic mass of Na = 22.99 g/mol; Atomic mass of Cl = 35.45 g/mol
Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
2. How many moles of magnesium are present in 25.0 g of magnesium oxide (MgO)?
Solution:Molar mass of MgO = 24.31 g/mol + 16.00 g/mol = 40.31 g/mol
Moles of Mg = 25.0 g / 40.31 g/mol = 0.620 mol
Applications of Unit Chemical Quantities and Molar Mass
Unit chemical quantities and molar mass have numerous applications in chemistry. These applications include:
- Stoichiometry:Unit chemical quantities and molar mass are used to balance chemical equations and determine the quantitative relationships between reactants and products.
- Chemical Reactions:Molar mass is used to calculate the amount of reactants and products involved in chemical reactions.
- Analytical Chemistry:Unit chemical quantities and molar mass are used in various analytical techniques, such as titration and gravimetric analysis.
Real-World Examples
- Determining the concentration of a solution.
- Calculating the amount of fertilizer needed for a field.
- Determining the purity of a substance.
Table of Unit Chemical Quantities and Molar Masses
| Unit Chemical Quantity | Symbol | Definition | Molar Mass (g/mol) |
|---|---|---|---|
| Mole | mol | Amount of substance containing as many elementary entities as there are atoms in 0.012 kilograms of carbon-12 | N/A |
| Gram | g | Mass of a substance | Varies |
| Liter | L | Volume of a substance | Varies |
| Milliliter | mL | One thousandth of a liter | Varies |
Further Exploration: Unit Chemical Quantities Molar Mass 2 Step Ws 3
For further exploration of unit chemical quantities and molar mass, refer to the following resources:
Answers to Common Questions
What is the difference between unit chemical quantities and molar mass?
Unit chemical quantities, such as the mole, represent specific amounts of substances, while molar mass, expressed in grams per mole, determines the mass of a given substance.
How is molar mass used in stoichiometry?
Molar mass is used in stoichiometry to determine the quantities of reactants and products involved in chemical reactions. By knowing the molar mass of each substance, chemists can calculate the mass or volume of reactants and products needed or produced in a reaction.