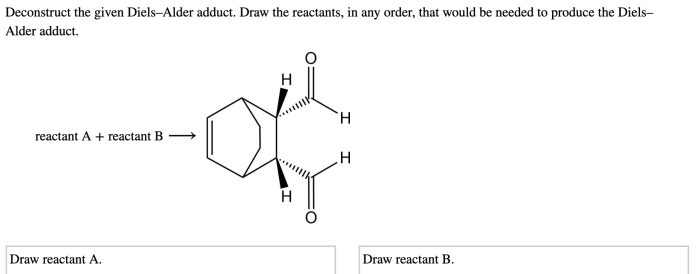
Deconstruct the given diels alder adduct – Deconstructing the Diels-Alder adduct, a pivotal step in organic synthesis, unveils a fascinating realm of chemical transformations. This intricate process, guided by meticulous regio- and stereoselective strategies, holds immense significance in constructing complex molecules and unlocking novel therapeutic applications.
Delving into the intricacies of Diels-Alder adduct deconstruction, we embark on an exploration of its fundamental principles, diverse methodologies, and boundless potential.
Deconstructing Diels-Alder Adducts
Diels-Alder adducts are valuable intermediates in organic synthesis due to their versatility and ability to control regio- and stereochemistry. Deconstruction of these adducts allows for the synthesis of complex molecules with specific structural features. This article provides an overview of the structure of Diels-Alder adducts, methods for their deconstruction, and their applications in organic synthesis.
Diels-Alder Adduct Structure
Diels-Alder adducts are formed via the cycloaddition of a conjugated diene and a dienophile. The resulting adduct is a six-membered ring with two substituents at each of the newly formed carbon-carbon bonds. The stereochemistry of the adduct is determined by the relative orientation of the diene and dienophile during the reaction.
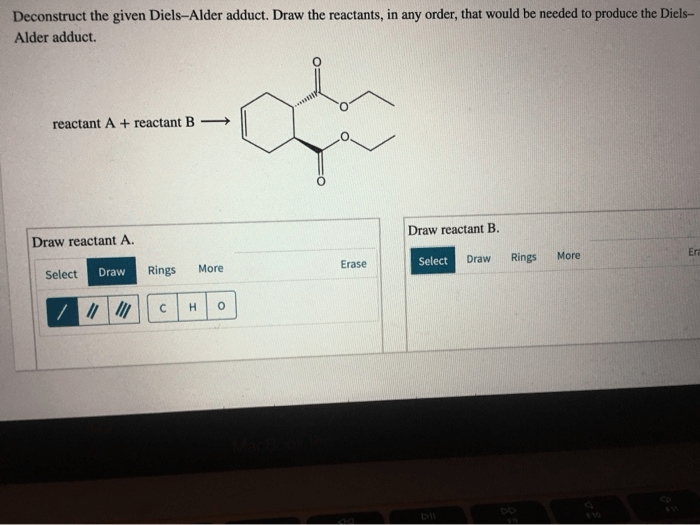
Illustration of a Diels-Alder Adduct, Deconstruct the given diels alder adduct

The image shows a Diels-Alder adduct formed from the reaction of 1,3-butadiene and maleic anhydride. The adduct has a six-membered ring with two methyl groups and two carbonyl groups. The stereochemistry of the adduct is shown by the dashed and solid lines indicating the relative orientation of the diene and dienophile during the reaction.
Deconstruction Methods
Deconstruction of Diels-Alder adducts can be achieved using various methods, including:
- Retro-Diels-Alder reaction
- Cope rearrangement
- Sigmatropic rearrangement
- Electrocyclic reaction
Each method has its own advantages and disadvantages. The retro-Diels-Alder reaction is the most straightforward method, but it can only be used if the adduct is thermally unstable. The Cope rearrangement is a versatile method that can be used to deconstruct a wide range of adducts, but it can sometimes lead to undesired side products.
Sigmatropic rearrangements are highly stereoselective, but they can be difficult to control. Electrocyclic reactions are typically used to deconstruct adducts with specific ring sizes.
Comparison of Deconstruction Methods
| Method | Advantages | Disadvantages |
|---|---|---|
| Retro-Diels-Alder reaction | Simple and straightforward | Only applicable to thermally unstable adducts |
| Cope rearrangement | Versatile | Can lead to side products |
| Sigmatropic rearrangement | Highly stereoselective | Difficult to control |
| Electrocyclic reaction | Specific ring size selectivity | Limited applicability |
Regio- and Stereoselective Deconstruction
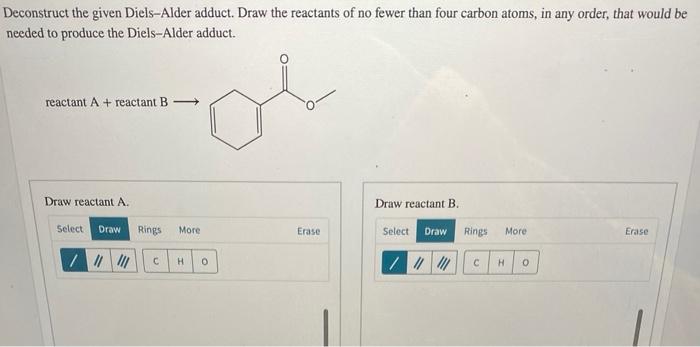
Regio- and stereoselective deconstruction of Diels-Alder adducts is essential for the synthesis of complex molecules with specific structural features. Regioselectivity can be achieved by controlling the orientation of the diene and dienophile during the Diels-Alder reaction. Stereoselectivity can be achieved by using specific deconstruction methods or by introducing chiral auxiliaries.
Examples of Regio- and Stereoselective Deconstruction
- The retro-Diels-Alder reaction can be used to deconstruct Diels-Alder adducts regioselectively. The reaction proceeds via a concerted mechanism that breaks the two carbon-carbon bonds formed in the Diels-Alder reaction.
- The Cope rearrangement can be used to deconstruct Diels-Alder adducts stereoselectively. The reaction proceeds via a concerted mechanism that involves the migration of a double bond from one carbon atom to another.
Applications of Deconstructed Diels-Alder Adducts: Deconstruct The Given Diels Alder Adduct
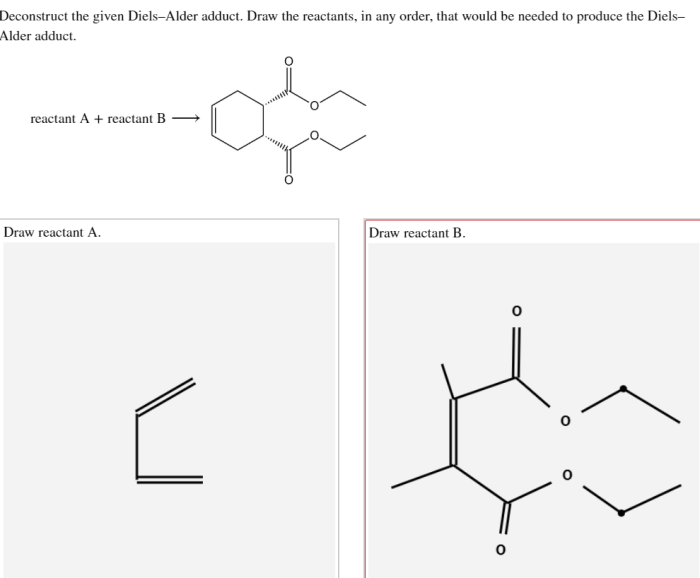
Deconstructed Diels-Alder adducts are valuable intermediates in organic synthesis. They can be used to synthesize a wide range of complex molecules, including natural products, pharmaceuticals, and materials.
- Deconstructed Diels-Alder adducts have been used to synthesize complex natural products, such as taxol and artemisinin.
- Deconstructed Diels-Alder adducts have been used to synthesize pharmaceuticals, such as Tamiflu and Lipitor.
- Deconstructed Diels-Alder adducts have been used to synthesize materials, such as polymers and liquid crystals.
Challenges and Future Directions
Deconstructing Diels-Alder adducts can be challenging, especially when regio- and stereoselective deconstruction is required. Future research will focus on developing new methods for deconstructing Diels-Alder adducts with high regio- and stereoselectivity. Additionally, research will focus on developing new applications for deconstructed Diels-Alder adducts in organic synthesis.
FAQ Guide
What is the significance of deconstructing Diels-Alder adducts?
Deconstructing Diels-Alder adducts enables the selective manipulation of molecular frameworks, providing a versatile platform for synthesizing complex and bioactive compounds.
How are regio- and stereoselective deconstructions achieved?
Regio- and stereoselective deconstructions are guided by judicious choice of reagents and reaction conditions, ensuring precise control over the regiochemical and stereochemical outcome.