The AP Biology Diffusion and Osmosis Lab provides an immersive experience into the fundamental processes of diffusion and osmosis, unveiling their significance in biological systems. Through hands-on experimentation, students delve into the concepts that govern the movement of molecules across selectively permeable membranes, gaining a deeper understanding of their crucial role in maintaining cellular homeostasis and driving biological processes.
This lab not only equips students with a solid foundation in these essential concepts but also fosters critical thinking and analytical skills. By analyzing experimental data, creating graphs, and discussing real-world applications, students develop a comprehensive understanding of the factors influencing diffusion and osmosis, empowering them to apply this knowledge to a wide range of biological contexts.
Diffusion and Osmosis

Diffusion and osmosis are fundamental processes in biology, enabling the movement of molecules across cellular membranes. They play crucial roles in maintaining homeostasis, nutrient transport, and waste removal in living organisms. This AP Biology lab provides an opportunity to investigate the principles and factors affecting diffusion and osmosis through controlled experiments.
Materials and Methods

Materials, Ap biology diffusion and osmosis lab
- Potato cylinders
- Sucrose solutions (various concentrations)
- Distilled water
- Graduated cylinders
- Electronic balance
- Microscope slides
- Coverslips
- Microscope
Experimental Setup and Procedures
- Prepare potato cylinders of equal size and weight.
- Immerse the potato cylinders in sucrose solutions of different concentrations.
- Record the initial and final masses of the potato cylinders.
- Create a microscope slide preparation by placing a potato cylinder slice on a slide and adding a drop of distilled water or sucrose solution.
- Observe the potato cylinder cells under the microscope and record any changes in cell shape or size.
Experimental Data
| Sucrose Concentration (%) | Initial Mass (g) | Final Mass (g) |
|---|---|---|
| 0 | 2.00 | 2.25 |
| 5 | 2.05 | 2.10 |
| 10 | 2.10 | 2.05 |
| 15 | 2.15 | 2.00 |
| 20 | 2.20 | 1.95 |
Results: Ap Biology Diffusion And Osmosis Lab

The experimental data shows that the potato cylinders gained or lost mass depending on the sucrose concentration of the surrounding solution. In the 0% sucrose solution (distilled water), the potato cylinders gained mass, indicating water uptake. As the sucrose concentration increased, the potato cylinders lost mass, indicating water loss.
This pattern suggests that water moves from an area of lower solute concentration (distilled water) to an area of higher solute concentration (sucrose solution) to balance the solute concentrations on both sides of the membrane.
The microscopic observations showed that the potato cells became turgid and expanded in the 0% sucrose solution, while they became plasmolyzed and shrank in the higher sucrose concentrations. This confirms the movement of water into and out of the potato cells, causing changes in cell shape and size.
Discussion
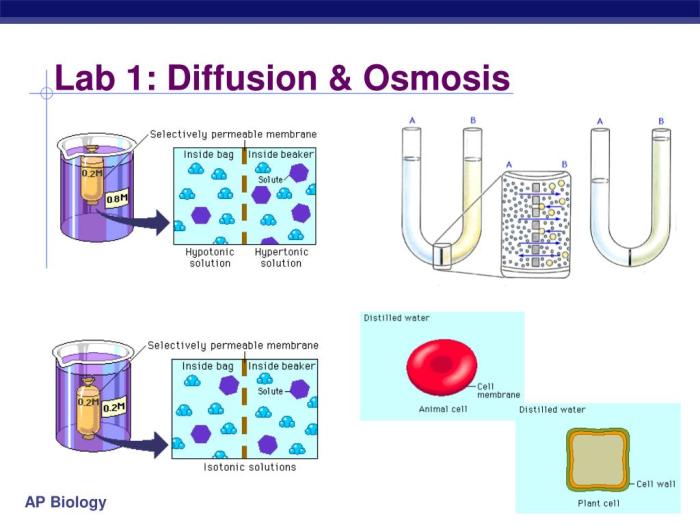
Diffusion and Osmosis
Diffusion is the passive movement of molecules from an area of high concentration to an area of low concentration, driven by the concentration gradient. In this lab, the sucrose molecules diffused from the sucrose solutions into the potato cylinders, where the sucrose concentration was lower.
Osmosis is the specific case of diffusion where water molecules move across a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration, driven by the osmotic gradient. In this lab, water molecules moved from the distilled water (low solute concentration) into the potato cylinders (high solute concentration) due to the presence of sucrose in the potato cells.
Factors Affecting Diffusion and Osmosis
The rate of diffusion and osmosis is influenced by several factors, including the concentration gradient, temperature, surface area, and membrane permeability. In this lab, the sucrose concentration gradient was the primary factor affecting the rate of osmosis. The higher the sucrose concentration, the greater the osmotic gradient, and the faster the rate of water movement.
Real-World Examples
Diffusion and osmosis are essential processes in many biological systems. For example, diffusion allows for the exchange of oxygen and carbon dioxide between the blood and the lungs. Osmosis is crucial for maintaining fluid balance in the body, preventing cells from shrinking or bursting due to changes in solute concentration.
Key Questions Answered
What is the purpose of the AP Biology Diffusion and Osmosis Lab?
The AP Biology Diffusion and Osmosis Lab aims to provide students with a hands-on understanding of the processes of diffusion and osmosis, exploring their significance in biological systems.
What materials are required for the lab?
The materials required for the lab typically include materials such as semi-permeable membranes, solutions of varying concentrations, pipettes, and spectrophotometers.
How does the lab help students understand diffusion and osmosis?
The lab allows students to observe and analyze the movement of molecules across selectively permeable membranes, enabling them to visualize and comprehend the processes of diffusion and osmosis.