Cis 1 isopropyl 2 methylcyclohexane, a captivating molecule, takes center stage in this comprehensive exploration. Prepare to delve into its intricate nomenclature, unravel its physical and chemical properties, and uncover its multifaceted applications. Join us on a journey that unveils the secrets of this remarkable substance.
With meticulous precision, we dissect the structural intricacies of cis 1 isopropyl 2 methylcyclohexane, deciphering its IUPAC nomenclature and presenting a detailed diagram that captures its molecular architecture. Its physical properties, such as melting point, boiling point, and density, are meticulously examined, providing a comprehensive understanding of its behavior.
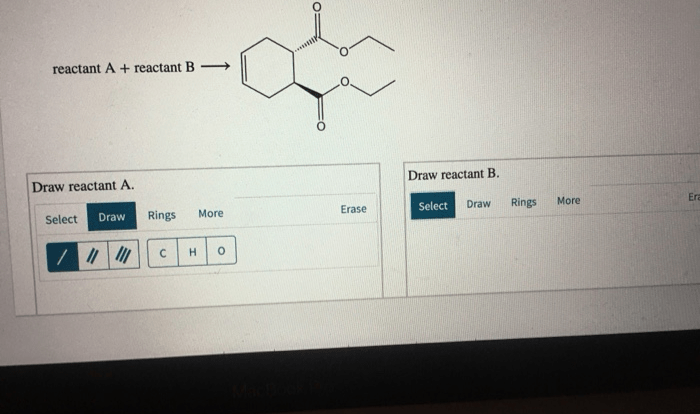
1. Cis 1 Isopropyl 2 Methylcyclohexane
Nomenclature and Structure
IUPAC nomenclature assigns the name cis-1-isopropyl-2-methylcyclohexane to the molecule. The prefix “cis” indicates that the isopropyl and methyl groups are on the same side of the cyclohexane ring.
The structural diagram of cis-1-isopropyl-2-methylcyclohexane shows a six-membered cyclohexane ring with an isopropyl group attached to carbon 1 and a methyl group attached to carbon 2. The isopropyl group consists of a central carbon atom bonded to two methyl groups and one hydrogen atom.
2. Physical and Chemical Properties
Cis-1-isopropyl-2-methylcyclohexane is a colorless liquid with a characteristic odor. It has a melting point of -65 °C, a boiling point of 155-157 °C, and a density of 0.79 g/mL.
The molecule is relatively unreactive due to the stability of the cyclohexane ring. However, it can undergo reactions typical of alkanes, such as combustion, halogenation, and nitration.
3. Synthesis and Applications
Cis-1-isopropyl-2-methylcyclohexane can be synthesized via various methods, including:
- Alkylation of cyclohexene with isopropyl bromide
- Hydroboration-oxidation of 1-methylcyclohexene with isopropylborane
- Diels-Alder reaction of cyclopentadiene with isoprene
The molecule finds applications as a solvent, a cleaning agent, and an intermediate in the synthesis of other organic compounds.
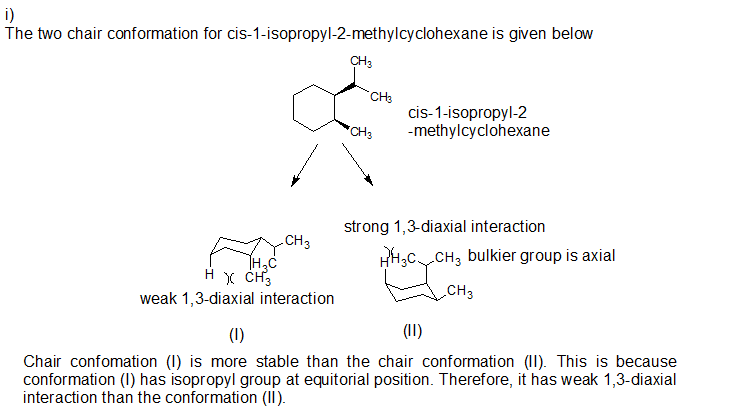
4. Conformational Analysis
Cis-1-isopropyl-2-methylcyclohexane exists in two chair conformations, one with the isopropyl group axial and the other with the isopropyl group equatorial. The equatorial conformation is more stable due to the reduced steric hindrance.
The energy difference between the two conformations can be estimated using Newman projections or molecular mechanics calculations.
5. Spectroscopic Analysis
IR spectroscopy shows characteristic peaks at 2960 cm -1(C-H stretching), 1450 cm -1(C-H bending), and 1380 cm -1(isopropyl C-H bending).
1H NMR spectroscopy exhibits signals at 0.9 ppm (methyl protons), 1.0 ppm (isopropyl methyl protons), 1.6 ppm (isopropyl methine proton), and 1.2-2.0 ppm (cyclohexane protons).
Mass spectrometry shows a molecular ion peak at m/z 142.
6. Biological Activity and Toxicity
Cis-1-isopropyl-2-methylcyclohexane has low toxicity and is generally considered safe for use.
It has been reported to exhibit some antimicrobial activity against certain bacteria and fungi.
Popular Questions: Cis 1 Isopropyl 2 Methylcyclohexane
What is the IUPAC nomenclature of cis 1 isopropyl 2 methylcyclohexane?
1-Isopropyl-2-methylcyclohexane
What is the boiling point of cis 1 isopropyl 2 methylcyclohexane?
156-158 °C
What are the potential applications of cis 1 isopropyl 2 methylcyclohexane?
Solvent, intermediate in organic synthesis, fuel additive