Vapor pressure and boiling worksheet is an essential tool for understanding the behavior of liquids and their phase transitions. This worksheet provides a comprehensive overview of vapor pressure, boiling point, vapor pressure curve, Clausius-Clapeyron equation, and vapor pressure measurement techniques.
Vapor pressure is a key property of liquids that influences their boiling point, evaporation rate, and many other physical and chemical processes. By understanding the factors that affect vapor pressure, scientists and engineers can design and optimize systems that involve liquid-vapor phase transitions.
Introduction
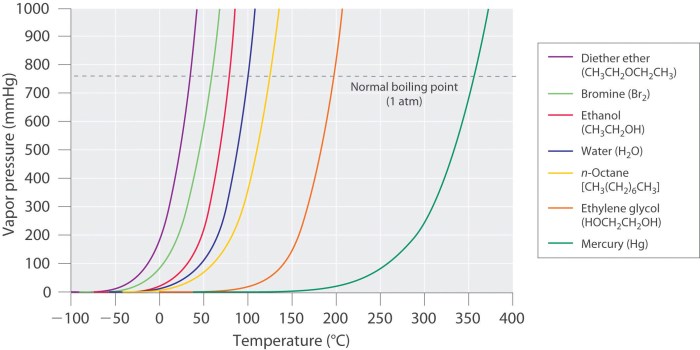
Vapor pressure is a critical concept in understanding the behavior of liquids. It refers to the pressure exerted by the vapor of a liquid when it is in equilibrium with its liquid phase. Vapor pressure plays a significant role in determining the boiling point, evaporation rate, and other properties of liquids.
Several factors influence vapor pressure, including temperature, molecular structure, and intermolecular forces. Understanding these factors is essential for comprehending the behavior of liquids in various applications.
Factors Influencing Vapor Pressure
Temperature has a direct impact on vapor pressure. As temperature increases, the kinetic energy of the molecules in the liquid increases, leading to a higher tendency to escape into the gas phase. Consequently, vapor pressure increases with temperature.
Molecular structure also influences vapor pressure. Liquids with smaller and less polar molecules generally have higher vapor pressures than those with larger and more polar molecules. This is because smaller molecules have weaker intermolecular forces, making it easier for them to escape into the gas phase.
Intermolecular forces play a crucial role in determining vapor pressure. Liquids with strong intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, have lower vapor pressures compared to liquids with weaker intermolecular forces. This is because stronger intermolecular forces hold the molecules together more tightly, making it more difficult for them to escape into the gas phase.
Boiling Point: Vapor Pressure And Boiling Worksheet
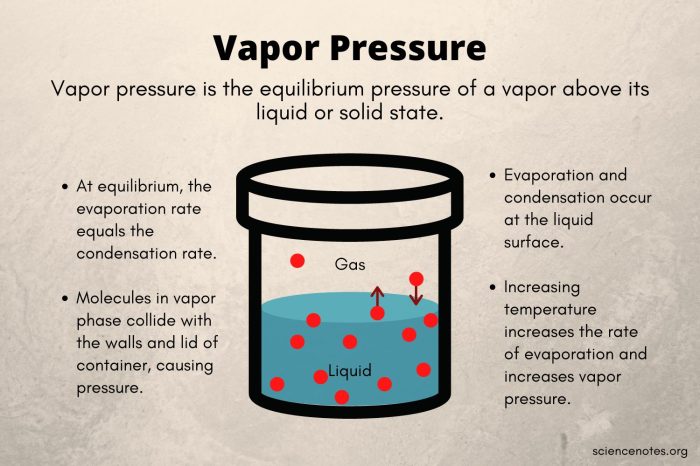
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid is an important physical property that is used to characterize the liquid. It is also used in many industrial and chemical processes.
Factors Affecting Boiling Point
The boiling point of a liquid is affected by several factors, including:
- Pressure: The boiling point of a liquid increases with increasing pressure.
- Molecular weight: The boiling point of a liquid increases with increasing molecular weight.
- Polarity: The boiling point of a liquid increases with increasing polarity.
- Impurities: The boiling point of a liquid is lowered by the presence of impurities.
Vapor Pressure Curve
The vapor pressure curve depicts the relationship between the vapor pressure of a substance and its temperature. It is a crucial tool for understanding the phase behavior of substances and their transitions between liquid and gas phases.
The vapor pressure curve can be divided into three distinct regions:
Liquid-Vapor Equilibrium Region
In this region, the vapor pressure of the liquid increases with temperature. The liquid and vapor phases coexist in equilibrium, meaning that the rate of evaporation is equal to the rate of condensation. This region lies below the critical point.
Critical Point
The critical point is a unique point on the vapor pressure curve where the liquid and gas phases become indistinguishable. At this point, the vapor pressure is equal to the pressure of the surrounding environment, and the substance exists as a supercritical fluid.
The critical point is denoted by Tcand Pc.
Supercritical Fluid Region
In this region, the substance exists as a supercritical fluid, which has properties of both a liquid and a gas. Supercritical fluids have high densities like liquids but can penetrate like gases. They are often used as solvents in chemical processes.
Clausius-Clapeyron Equation
The Clausius-Clapeyron equation is a thermodynamic equation that relates the vapor pressure of a liquid to its temperature. It is named after Rudolf Clausius and Benoit Paul Emile Clapeyron, who independently derived the equation in the 19th century.The Clausius-Clapeyron equation can be used to determine the enthalpy of vaporization of a liquid.
The enthalpy of vaporization is the amount of heat required to convert one mole of a liquid into a gas at its boiling point.
Calculating the Enthalpy of Vaporization
To calculate the enthalpy of vaporization using the Clausius-Clapeyron equation, the following steps are taken:
- Measure the vapor pressure of the liquid at two different temperatures.
- Plot the vapor pressure data on a graph of ln(P) versus 1/T.
- Calculate the slope of the line.
4. Use the slope of the line to calculate the enthalpy of vaporization using the following equation
“`ΔHvap =
- R
- slope
“`where:* ΔHvap is the enthalpy of vaporization
- R is the ideal gas constant (8.314 J/mol*K)
- slope is the slope of the line on the graph of ln(P) versus 1/T
Examples
The following are examples of how to use the Clausius-Clapeyron equation to calculate the enthalpy of vaporization of different substances:*
-*Water
The enthalpy of vaporization of water at 100 °C is 40.7 kJ/mol.
-
-*Ethanol
The enthalpy of vaporization of ethanol at 78.5 °C is 38.6 kJ/mol.
-*Benzene
The enthalpy of vaporization of benzene at 80.1 °C is 30.7 kJ/mol.
Vapor Pressure Measurement
Measuring vapor pressure is crucial for understanding the behavior of substances and their interactions with their surroundings. Various methods have been developed to accurately determine vapor pressure, each with its own advantages and limitations.
Manometer Method
The manometer method involves connecting a closed vessel containing the liquid to a manometer. As the liquid evaporates, the pressure inside the vessel increases, causing the manometer fluid to move. The difference in height between the manometer fluid levels is directly proportional to the vapor pressure of the liquid.
Advantages:
- Simple and straightforward to use
- Can be used for a wide range of liquids
Disadvantages:
- Requires a closed vessel, which may not be suitable for all liquids
- Can be affected by temperature fluctuations
Dew Point Method, Vapor pressure and boiling worksheet
The dew point method measures the temperature at which water vapor in the air condenses into liquid water. This temperature is known as the dew point and is directly related to the vapor pressure of water in the air.
Advantages:
- Can be used to measure vapor pressure in gases
- Non-invasive and does not require a closed vessel
Disadvantages:
- Only applicable to water vapor
- Can be affected by the presence of other gases in the air
Applications of Vapor Pressure
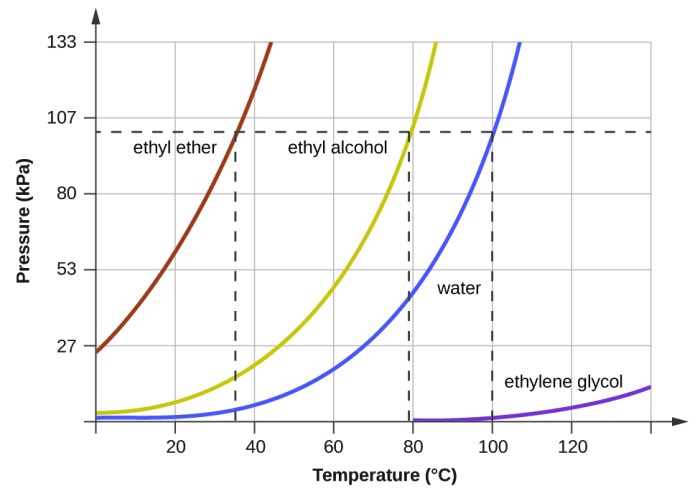
Vapor pressure finds diverse applications in various scientific and industrial fields, providing valuable insights into the behavior of substances and enabling the optimization of processes.
In chemistry, vapor pressure data is crucial for determining the purity of substances, predicting reaction rates, and designing distillation and evaporation processes. By measuring the vapor pressure of a liquid mixture, it is possible to determine the composition of the mixture and identify the presence of impurities.
Engineering Applications
- In engineering, vapor pressure is essential for designing and optimizing heat transfer systems, such as boilers, condensers, and evaporators. By understanding the vapor pressure of the working fluid, engineers can optimize the efficiency and safety of these systems.
- Vapor pressure also plays a vital role in the design of refrigeration and air conditioning systems. The vapor pressure of the refrigerant determines the operating conditions of the system, including the compressor size, evaporator temperature, and condenser pressure.
Environmental Science Applications
- In environmental science, vapor pressure data is used to assess the fate and transport of pollutants in the atmosphere and water bodies. By measuring the vapor pressure of a pollutant, scientists can predict its volatility and its potential to evaporate into the environment.
- Vapor pressure is also important for understanding the behavior of pesticides and other chemicals in soil and water. By knowing the vapor pressure of a chemical, it is possible to estimate its potential to volatilize and contaminate the environment.
Frequently Asked Questions
- In environmental science, vapor pressure data is used to assess the fate and transport of pollutants in the atmosphere and water bodies. By measuring the vapor pressure of a pollutant, scientists can predict its volatility and its potential to evaporate into the environment.
- Vapor pressure is also important for understanding the behavior of pesticides and other chemicals in soil and water. By knowing the vapor pressure of a chemical, it is possible to estimate its potential to volatilize and contaminate the environment.
Frequently Asked Questions
What is vapor pressure?
Vapor pressure is the pressure exerted by the vapor of a liquid when it is in equilibrium with its liquid phase.
How does temperature affect vapor pressure?
Vapor pressure increases with increasing temperature.
What is the boiling point of a liquid?
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure of the surrounding environment.
What is the Clausius-Clapeyron equation?
The Clausius-Clapeyron equation is a thermodynamic equation that relates the vapor pressure of a liquid to its temperature and enthalpy of vaporization.
How is vapor pressure measured?
Vapor pressure can be measured using various methods, including the manometer method and the dew point method.